3 min read
Jan 22, 2025
The Pharmaceutical Distribution Journey in Pakistan: From Manufacturer to Pharmacy Shelf
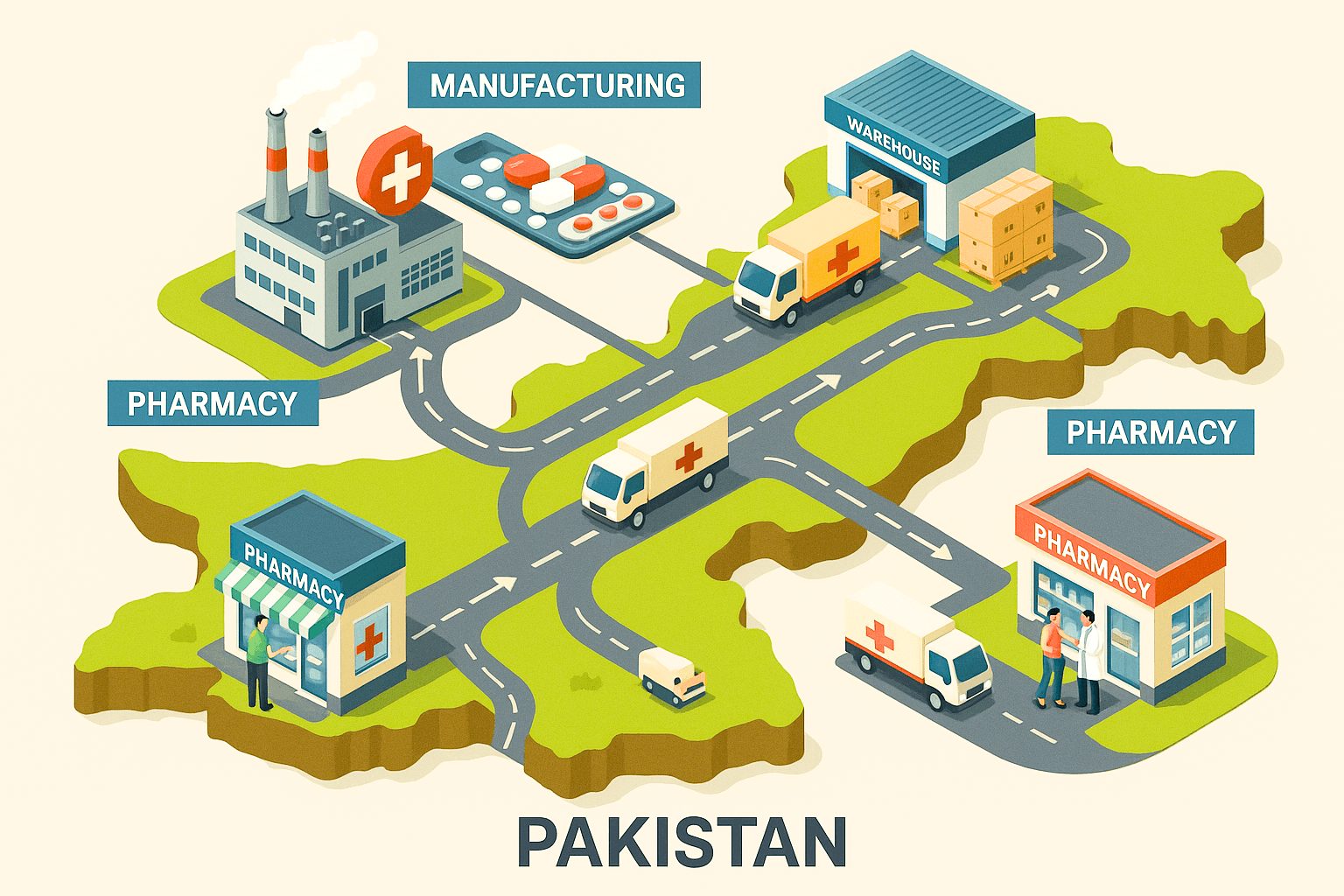
The pharmaceutical distribution system in Pakistan is a vital link connecting medicine manufacturers with healthcare providers and patients across the country. Ensuring medicines are delivered safely, on time, and in optimal condition requires a coordinated effort across manufacturing, warehousing, transportation, and retail stages.
Understanding this journey helps pharmaceutical companies, pharmacies, and stakeholders optimize their supply chains, ensure regulatory compliance, and ultimately improve patient care.
1. Manufacturing: The Foundation of Quality Medicines
Pharmaceutical manufacturing in Pakistan operates under strict regulations governed by the Drug Regulatory Authority of Pakistan (DRAP). This includes:
Good Manufacturing Practices (GMP) compliance
Rigorous quality testing at every batch stage
Proper packaging with required labeling and safety features
The process ensures that only safe, effective, and high-quality medicines enter the distribution chain.
2. Warehousing: Safeguarding Medicine Integrity
Post-manufacturing, medicines are stored in warehouses that must maintain optimal conditions. Many medicines, especially vaccines, insulin, and biologics, require temperature-controlled environments known as the cold chain to preserve efficacy.
Key warehouse features include:
Temperature and humidity controls
Backup power systems to prevent spoilage during outages
Inventory management systems to track stock levels and expiry dates
Reliable warehousing prevents medicine degradation and supports regulatory compliance.
3. Distribution: The Critical Link
Distributors bridge the gap between manufacturers and healthcare providers. They manage complex logistics to ensure timely and safe medicine delivery.
Role of Local and Regional Distributors
Pharmaceutical distribution in Pakistan is often regionally organized due to the country’s size and diverse infrastructure. Local distributors are essential because they:
Understand regional demand patterns and healthcare infrastructure
Provide rapid response for urgent orders
Navigate local regulatory and logistical challenges
National and regional distributors work in tandem to extend medicine reach from urban centers to remote areas.
Compliance and Quality Assurance
Distributors must adhere to DRAP’s Good Distribution Practices (GDP), ensuring:
Proper storage and handling during transport
Use of validated temperature-controlled vehicles for sensitive products
Full traceability through electronic documentation and batch records
These practices are crucial for maintaining medicine safety and patient trust.
4. Pharmacy Shelves: The End Point of Distribution
Pharmacies and healthcare facilities are the final recipients in the distribution chain. Their role includes:
Managing stock efficiently to prevent shortages or expired medicines
Dispensing medicines with proper counseling to patients
Maintaining compliance with DRAP licensing and record-keeping requirements
Consistent supply from reliable distributors ensures pharmacies can serve their communities effectively.
5. Challenges in Pharmaceutical Distribution in Pakistan
The supply chain faces various hurdles:
Infrastructure Limitations: Road conditions and transport logistics can delay deliveries, especially in remote areas.
Cold Chain Management: Maintaining temperature-sensitive medicines in Pakistan’s hot climate requires investment in technology and training.
Counterfeit Medicines: Preventing counterfeit and substandard medicines requires vigilance throughout the supply chain.
Regulatory Complexity: Keeping up with evolving DRAP regulations demands ongoing compliance efforts from all stakeholders.
6. The Impact of Technology on Modern Pharma Distribution
Technology is transforming Pakistan’s pharmaceutical distribution by enabling:
Advanced Inventory Management: Systems like Oracle-based software optimize stock control and demand forecasting.
Real-time Tracking: GPS-enabled delivery vehicles provide transparency and timely updates to pharmacies.
Electronic Documentation: Digital batch records ensure traceability and ease regulatory audits.
Leading distributors incorporate these technologies to improve efficiency and reliability.
7. Why Choosing the Right Distribution Partner Matters
Pharmaceutical companies and pharmacies must choose distributors who combine:
Strong local and regional market knowledge
Robust infrastructure, including temperature-controlled storage
Commitment to regulatory compliance and ethical practices
Investment in technology for tracking and inventory management
Proven ability to handle emergency and last-mile deliveries
Companies like Ross Pharmaceuticals, with decades of experience and advanced logistics capabilities, exemplify the type of partner needed to navigate Pakistan’s complex pharmaceutical distribution landscape successfully.